Music and How it Helps You Stay Focus
Attention, concentration, focus, flow… These are words associated with mental states that assist in doing work. If we can focus our attention, we might be able to concentrate long enough to reach flow state, and actually get some work done. In this stimulation-rich world, focusing attention and finding flow is more easily discussed than done, but the scientific understanding of attentional processes can simplify the challenge.
Our senses are constantly inundated with information: the light streaming in the windows, the sight of people passing by on the street, the smells of a cafe, the sounds of conversations and dogs barking over the din of city traffic, the light scrape of oxford cloth around your neck, the pressure of the hardwood table on your elbows. Each time you notice something in your environment, you are paying attention to it. The ability to focus your attention on something while ignoring competing stimuli is called selective attention by psychologists, and we would never get anything done without it. Can you imagine how distracting it would be to notice every little detail in your environment at all times?
Selective attention has been likened to a spotlight that you focus on something, and as a spotlight, the beam can be wide or narrow. Right now, your spotlight is focused on this article, which means that it is probably rather narrow. Other aspects of your environment fade into the background in relation to what is in your attentional spotlight. Until, something distracting happens in your environment, and refocuses your spotlight.
The question many people want to be answered is how they can maximize focus so that their environment becomes less distracting. Neuroscience and psychology give us clues to help answer this question.
Evolution over millions of years gave humans a brain that is well-suited to life in small, nomadic, hunter-gatherer social groups. Our eyes are our most important asset, as evidenced by the amount of neural real-estate they occupy… nearly one-third of our brains are dedicated to vision. We are capable of detecting a single photon of light hitting our retina, as well as stealthy predators camouflaged against the grassy savannah, or prey hiding in the trees. Our ears can translate sounds between 20 – 20,000 Hz, which is MARVELOUS for making out the distinct calls of birds or the hushed voices of our tribal neighbors sounding low over the evening fire. Our somatic senses warn us when a tick brushes past a hair on our neck on its search for a meal, or just as importantly when our bodies are running low on fuel.
for making out the distinct calls of birds or the hushed voices of our tribal neighbors sounding low over the evening fire. Our somatic senses warn us when a tick brushes past a hair on our neck on its search for a meal, or just as importantly when our bodies are running low on fuel.
 for making out the distinct calls of birds or the hushed voices of our tribal neighbors sounding low over the evening fire. Our somatic senses warn us when a tick brushes past a hair on our neck on its search for a meal, or just as importantly when our bodies are running low on fuel.
for making out the distinct calls of birds or the hushed voices of our tribal neighbors sounding low over the evening fire. Our somatic senses warn us when a tick brushes past a hair on our neck on its search for a meal, or just as importantly when our bodies are running low on fuel.
Today, we don’t necessarily have to worry about predators, and prey is often found with a quick stop at the local grocer’s freezer aisle. But, the neural equipment we evolved to detect, process, and respond to environmental stimuli is still the same. Information is transformed from its particular modality (sight, sound, touch, etc.) into the electrochemical language of the nervous system by sensory cells throughout the body. For example, when you listen to music, sound waves hit your eardrum, and are transferred to the cochlea in your inner ear, where microscopic cells called hair cells are made to vibrate. The movement of the hair cells turns the mechanical energy of the wave into chemical signals that stimulate auditory nerves to fire action potentials.
Where does the signal go from there? The auditory pathway takes the encoded action potential signal from the ears, down cranial nerve 8, the vestibulocochlear nerve, to the brainstem where it synapses with nerves in the cochlear nucleus. This is the first location in the nervous system where signal processing takes place, and the signal becomes more defined. From here, the signal disperses down a neural expressway called the lateral lemniscus, which connects the cochlear nucleus to many other brainstem nuclei, including the superior olivary nucleus and the reticular formation, and eventually the inferior colliculus in the pons. Finally, the signal moves from the Inferior colliculus to the thalamus, and onward to the primary auditory cortex in the temporal lobe.
The preceding paragraph is loaded with very specific information, and you’re probably wondering why knowing the path of information related to sound within the nervous system has anything to do with attention. Well, scientists have not only named regions within the brain with hard-to-remember names, they have also done a lot of experiments to figure out what the regions do. In fact, there is an entire branch of psychology, called psychoacoustics, dedicated to the study of the perception of sound (mainly speech and music).
Most brainstem-level signal processing has to do with the localization of sound. But, we also know that when information gets into the brainstem, one of the areas that are likely to be activated is a nucleus of monoamine-producing neurons called the locus coeruleus. Monoamines are used in the brain as neurotransmitters or chemicals that send messages from one neuron to another. In the case of the locus coeruleus, it mainly produces the monoamine neurotransmitter known as noradrenaline or norepinephrine, which is a stimulant for your brain, activating the sympathetic part of your nervous system.
Activation of the locus coeruleus triggers the release of norepinephrine at targets all over your brain, including the thalamus, hippocampus, amygdala, and frontal cortex. The areas it targets, many of which are part of the limbic system, are responsible for deciding how you are going to respond behaviorally to a stimulus, which in this case is music. In effect, by dousing the decision-making parts of your brain with a stimulant, the locus coeruleus increases your level of arousal from the bottom up and mediates behavioural responses so that you will be either more or less distractible.
But, although researchers have suggested that dysregulation of the locus coeruleus likely contributes to many arousal disorders, such as insomnia, anxiety, ADHD, DEPRESSION , and PTSD[2, 3], it’s much more complicated than just stimulus-increasing arousal. If that were the case, the majority of us would always be way too stimulated.
, and PTSD[2, 3], it’s much more complicated than just stimulus-increasing arousal. If that were the case, the majority of us would always be way too stimulated.
 , and PTSD[2, 3], it’s much more complicated than just stimulus-increasing arousal. If that were the case, the majority of us would always be way too stimulated.
, and PTSD[2, 3], it’s much more complicated than just stimulus-increasing arousal. If that were the case, the majority of us would always be way too stimulated.
We also have top-down control called executive attention: the frontal cortices of our brains allow us to manage our attention the way that a teacher manages a classroom. Instead of allowing the students in the class to all talk at once, creating an unbearable din, the teacher determines how many and who of the students get to talk. So, when you sit down at that table in the cafe to read, your forebrain can send signals to the locus coeruleus telling it to keep quiet, except in the case of an emergency. This feedback loop allows you to focus your attention on your book for a sustained period of time, rather than being constantly distracted by everything going on in the cafe.
Interestingly, scientists have been able to determine specific peaks in brain activity, called event-related potentials (ERPs), using both electro- (EEG) and magneto-encephalography (MEG) technologies that coincide with the activation of different brain areas and the different stages of attention. First, there are early ERPs that occur just 50-100ms after the onset of a stimulus and signify early processing and unconscious detection. ERPs occurring 300-800ms after stimulus onset are thought to relate to higher-level processing, conscious recognition of the stimulus, and unconscious decision-making, or detection of an unexpected stimulus. 180ms later your personal reaction time determines how long it will take you to fully react to the stimulus if you choose to do so.[5,6] It’s amazing that so much can happen in a little more time than it takes to blink your eye.
An ERP called P300 has been used as a measure of cognitive function[20, 21, 22], and appears to increase in amplitude when the brain detects an unexpected or oddball stimulus[6]. The amplitude effect is pronounced when one’s attention is selectively focused on something else, and likely reflects the need to redirect your attention when something unexpected occurs in your environment. In the cafe, where you are deeply engrossed in your book, you notice when a cup is dropped on the floor and breaks, or when someone walks up to your table and says your name. The time it takes your brain to notice these things and integrate the information about them related to your various sensory modalities determines how long it takes for you to respond.
However, although cafes are full of noises of all sorts, many people find it easier to work in a loud cafe than in a quiet library. Some theorists say this phenomenon is the result of cognitive load; your brain only has so much processing power for any given sensory modality at a time. The overloading of your auditory senses with stimuli results in a process wherein over a short period of time, usually about 20 minutes or so[9], you get used to the noise. This process is called habituation, and because of it, you are able to free up processing power for the task at hand. You will probably still notice when something unexpected pops up, but the neurons in your brain responsible for helping you sense the stuff in your environment quiet down and let you focus.[7] This is also what happens within your brain when you are at a cocktail party and try to listen selectively to one person over the sounds of the entire room, and was elegantly first described as the “Cocktail Party Problem” back in the 1950s [8].
So, it seems part of the trick is occupying your brain just enough to let you work. In this vein, it has been shown that listening to music while you work can do the trick[26]. Of course, some music is better than others: music that has emotional or sentimental overtones is likely to stimulate your amygdala (emotions) and hippocampus (memory) in your limbic system, and music that is too fast, variable, or loud will jar your locus coeruleus back into action[10, 12]. Huang and Shih (2011) found that when choosing music for a workplace, it is best to use music that workers neither like nor dislike[13]. In 2012, the same researchers concluded that music with lyrics is distracting to workers when compared to instrumental music[14]. Again, cognitive load theory dictates that if a person is attending to a task using multiple modalities, like reading, which depends on both the visual and auditory cortices, processing power will not be sufficient to ignore distractions in one of the modalities, like speech.
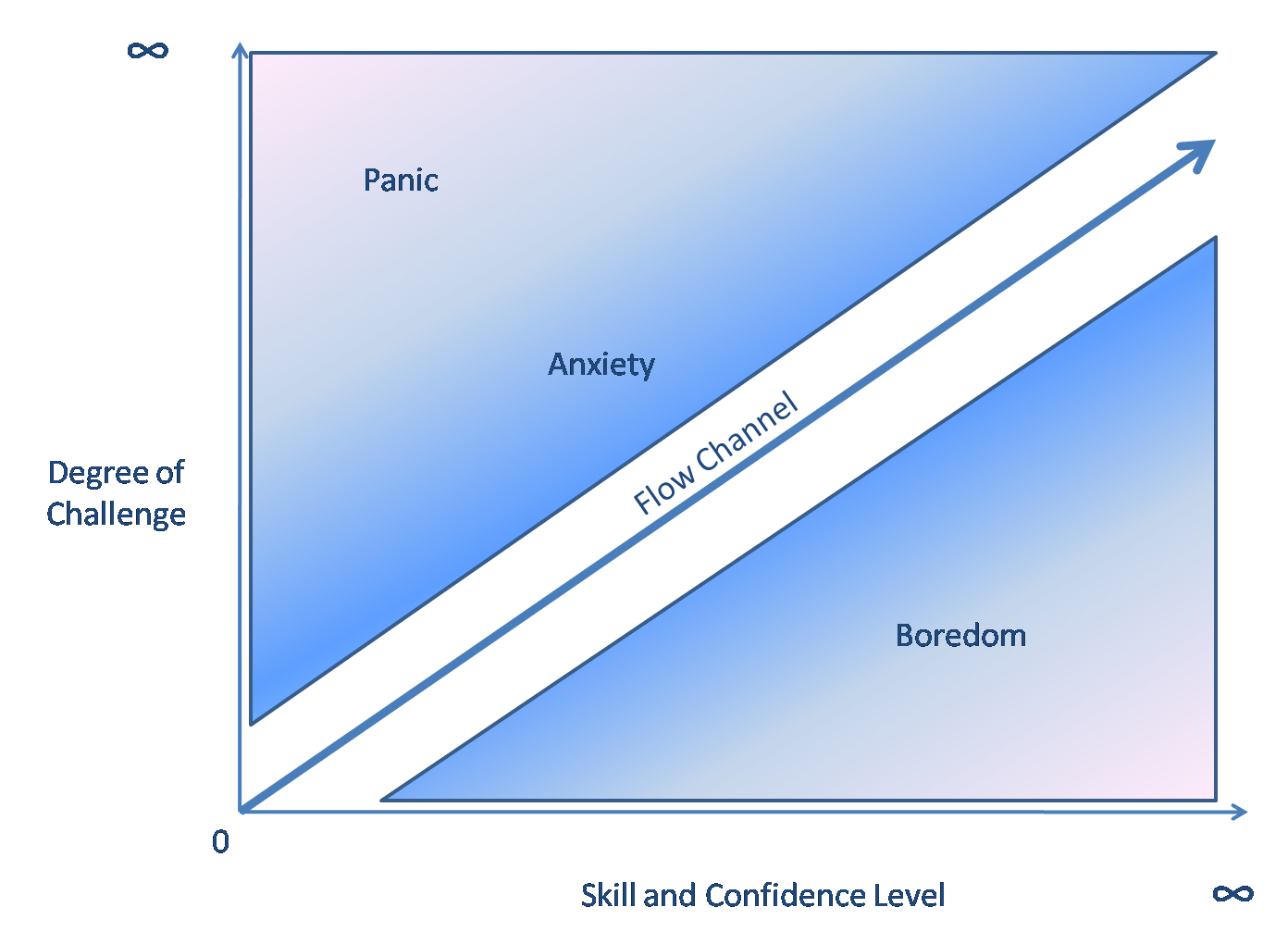
Listening to music with soothing aspects, that play at 60 beats per minute, can decrease neural activity, and lead to a relaxed, but awake state called alpha state[11] which is defined by an increase in alpha brain waves and a decrease in higher activity beta waves. Increases in alpha waves have been tied to a psychological state of decreased self-awareness, timelessness, and motivation known as “flow”. Songwriters, musicians, writers, athletes, and meditators are all people who separately describe the same experience when the flow state is reached.[15]
Once the flow state is reached how is it maintained, and for how long? We know that it takes approximately 20 minutes to get into a state of concentration, flow or not, in which you are able to habituate to irrelevant external stimuli[9]. Most people are able to maintain their concentration from a minimum of 20 to a maximum of about 40 minutes before having to take a break[16, 17]. Psychologists call this waning of attention the ‘vigilance decrement’, and suggest that it is due to either a reduction in cognitive resources or mindlessness and goal habituation.[18] Some research suggests that a brief break can reduce this goal habituation and enable people to maintain vigilance for longer periods of time.[19]
Exactly how long is still in question, but endocrinologists and brain researchers have described hormone and activity cycles that take place on the order of every 60 to 120 minutes[20, 22, 24, 25]. Based on the understanding that our bodies have natural daily rhythms and that these rhythms are tied to neural activity and cognitive function[22], work sessions could be organized to follow your increases and decreases in hormones, neurotransmitters, and cognitive function. Depending upon your personal daily rhythm, this would indicate that being able to maintain attention during work sessions of 1-2 hours in length is optimal.
The entire process of maintaining focus for an extended period of time is not easy. It requires work on the part of your brain and is stressful[27]. An area of research devoted to designing technologies that work with your brain to make work less effortful is called neuroergonomics.[28, 29, 30] Through neuroergonomics, technologies can produce experiences that enable your brain to feel less stressed, and you get more work done. That said you still need to be personally motivated to get to work and find your flow.[31, 32, 33, 34]
It’s not going to happen on its own.
Footnotes
1. Sara, S. J., & Bouret, S. (2012). Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron. 76(1): 130-141.
2. Aston-Jones, G., Rajkowski, J., & Cohen, J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biological psychiatry. 46(9): 1309-1320.
3. Aston-Jones, G., Gonzalez, M., & Doran, S. (2007) Role of the locus coeruleus-norepinephrine system in arousal and circadian regulation of the sleep–wake cycle. In: Brain Norepinephrine: Neurobiology and Therapeutics, ed. Gregory A. Ordway, Michael A. Schwartz and Alan Frazer. Cambridge University Press.
4. Berridge, C. W. (2008). Noradrenergic modulation of arousal. Brain Res Rev. 58(1):1-17.
5. Makinen, V., May, P., & Tiitinen, H. (2004). Transient brain responses predict the temporal dynamics of sound detection in humans. NeuroImage. 21(2): 701-706.
6. Coull, J.T. (1998) Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 55(4): 343-361.
7. Lavie, N., Hirst, A., de Fockert, J. W., & Viding, E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology. 133(3): 339–54.
8. Cherry, E. C. (1953). Some experiments on the recognition of speech, with one and two ears. Journal of the Acoustic Society of America. 25:975-979.
9. Banbury, S., & Berry, D. C. (1997). Habituation and dishabituation to speech and office noise. Journal of Experimental Psychology: Applied. 3(3): 181-195.
10. Eldar, E., Ganor, O., Admon, R., Bleich, A., & Hendler, T. (2007). Feeling the Real World: Limbic Response to Music Depends on Related Content. Cereb. Cortex. 17 (12): 2828-2840.
11. Vijayalakshmi, K., Sridhar, S., & Khanwani, P. Estimation of effects of alpha music on EEG components by time and frequency domain analysis. (2010). Computer and Communication Engineering ICCCE 2010 International Conference.
12. Dalton, B. H. (2006). The effects of sound types and volumes on simulated driving performance, simple vigilance and heart rate. ProQuest Dissertations and Theses. Memorial University of Newfoundland (Canada).
13. Huang, R. H & Shih, Y. N. (2011). Effects of background music on the concentration of workers. Work. 38(4):383-7.
14. Shih, Y. N., Huang, R. H., & Chiang, H. Y. (2012) Background music: effects on attention performance. Work. 42(4):573-8.
15. Vlachopoulos, S. P., Karageorghis, C. I., & Terry, P. C. (2000). Hierarchical confirmatory factor analysis of the Flow State Scale in exercise. Journal of Sports Sciences. 18(10): 815-823.
16. Mackworth, N.H. (1948). The breakdown of vigilance during prolonged visual search. Q. J. Exp. Psychol. 1:6–21.
17. Eysenck MW. (1982). Attention and arousal. In: Cognition and performance. Berlin: Springer-Verlag.
18. Pattyn, N., Neyt, X., Henderickx, D., & Soetens, E. (2008). A psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue?. Physiology & Behavior. 93(1): 369-378.
19. Ariga, A., & Lleras, A. (2011). Brief and rare mental “breaks” keep you focused: Deactivation and reactivation of task goals preempt vigilance decrements. Cognition, 118(3), 439–443.
20. Klein, R. & Armitage, R. (1979). Rhythms in human performance: 1 1/2-hour oscillation in cognitive style. Science. 204(4399): 1326-1328.
21. Hansenne, M. (2000). The P300 event-related potential. II. Interindividual variability and clinical application in psychopathology. Clinical Neurophysiol. 30(4): 211-231.
22. Polich, J. (1997). On the relationship between EEG and P300: individual differences, aging, and ultradian rhythms. International Journal of Psychophysiology. 26(1–3): 299-317.
23. Donchin, E., Coles, M.G.H. (1988). Is the P300 component a manifestation of context updating?. Brain Behav. Sci. 11: 357-374.
24. Okawa, M., Matousek, M., Petersen, I. (1984) Spontaneous vigilance fluctuations in the daytime. Psychophysiology. 21: 207-211.
25. Tsuji, Y. Kobayashi, T. (1988). Short and long ultradian EEG components in daytime arousal. Electroenceph. Clin. Neurophysiol. 70: 110-117.
26. Lesiuk, T. (2005). The effect of music listening on work performance. Psychology of Music. 33(2): 173-191.
27. Warm JS, Parasuraman R, Matthews G. (2008). Vigilance requires hard mental work and is stressful. Hum. Factors. 50(3): 433-41.
28. Parasuraman, R., & Hancock, P. A. (2004). Neuroergonomics – Harnessing the power of brain science for human factors and ergonomics. Bulletin of the Human Factors and Ergonomics Society, December 2.
29. Parasuraman, R. (2011). Neuroergonomics: Brain, Cognition, and Performance at Work. Current Directions in Psychological Science, 20(3), 181–186. doi:10.1177/0963721411409176
30. Parasuraman, R. (2005). Neuroergonomics: An overview of research and applications. Foundations of Augmented Cognition Vol 11, 839–840.
31. Weber, R., Tamborini, R., Westcott‐Baker, A., & Kantor, B. (2009). Theorizing flow and media enjoyment as cognitive synchronization of attentional and reward networks. Communication Theory, 19(4), 397-422.
32. Ericsson, K. A., Krampe, R. Th., & Tesch-Römer, C. (1993). The role of deliberate practice in the acquisition of expert performance. Psychological Review, 100(3), 363-406.
33. Parasuraman, R., Nestor, P., & Greenwood, P. (1989). Sustained-attention capacity in young and older adults. Psychology and Aging, 4(3), 339-345.
34. Baumann, N., & Scheffer, D. (2011). Seeking flow in the achievement domain: The achievement flow motive behind flow experience. Motivation and Emotion,35(3), 267-284.
1. Sara, S. J., & Bouret, S. (2012). Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron. 76(1): 130-141.
2. Aston-Jones, G., Rajkowski, J., & Cohen, J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biological psychiatry. 46(9): 1309-1320.
3. Aston-Jones, G., Gonzalez, M., & Doran, S. (2007) Role of the locus coeruleus-norepinephrine system in arousal and circadian regulation of the sleep–wake cycle. In: Brain Norepinephrine: Neurobiology and Therapeutics, ed. Gregory A. Ordway, Michael A. Schwartz and Alan Frazer. Cambridge University Press.
4. Berridge, C. W. (2008). Noradrenergic modulation of arousal. Brain Res Rev. 58(1):1-17.
5. Makinen, V., May, P., & Tiitinen, H. (2004). Transient brain responses predict the temporal dynamics of sound detection in humans. NeuroImage. 21(2): 701-706.
6. Coull, J.T. (1998) Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 55(4): 343-361.
7. Lavie, N., Hirst, A., de Fockert, J. W., & Viding, E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology. 133(3): 339–54.
8. Cherry, E. C. (1953). Some experiments on the recognition of speech, with one and two ears. Journal of the Acoustic Society of America. 25:975-979.
9. Banbury, S., & Berry, D. C. (1997). Habituation and dishabituation to speech and office noise. Journal of Experimental Psychology: Applied. 3(3): 181-195.
10. Eldar, E., Ganor, O., Admon, R., Bleich, A., & Hendler, T. (2007). Feeling the Real World: Limbic Response to Music Depends on Related Content. Cereb. Cortex. 17 (12): 2828-2840.
11. Vijayalakshmi, K., Sridhar, S., & Khanwani, P. Estimation of effects of alpha music on EEG components by time and frequency domain analysis. (2010). Computer and Communication Engineering ICCCE 2010 International Conference.
12. Dalton, B. H. (2006). The effects of sound types and volumes on simulated driving performance, simple vigilance and heart rate. ProQuest Dissertations and Theses. Memorial University of Newfoundland (Canada).
13. Huang, R. H & Shih, Y. N. (2011). Effects of background music on the concentration of workers. Work. 38(4):383-7.
14. Shih, Y. N., Huang, R. H., & Chiang, H. Y. (2012) Background music: effects on attention performance. Work. 42(4):573-8.
15. Vlachopoulos, S. P., Karageorghis, C. I., & Terry, P. C. (2000). Hierarchical confirmatory factor analysis of the Flow State Scale in exercise. Journal of Sports Sciences. 18(10): 815-823.
16. Mackworth, N.H. (1948). The breakdown of vigilance during prolonged visual search. Q. J. Exp. Psychol. 1:6–21.
17. Eysenck MW. (1982). Attention and arousal. In: Cognition and performance. Berlin: Springer-Verlag.
18. Pattyn, N., Neyt, X., Henderickx, D., & Soetens, E. (2008). A psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue?. Physiology & Behavior. 93(1): 369-378.
19. Ariga, A., & Lleras, A. (2011). Brief and rare mental “breaks” keep you focused: Deactivation and reactivation of task goals preempt vigilance decrements. Cognition, 118(3), 439–443.
20. Klein, R. & Armitage, R. (1979). Rhythms in human performance: 1 1/2-hour oscillation in cognitive style. Science. 204(4399): 1326-1328.
21. Hansenne, M. (2000). The P300 event-related potential. II. Interindividual variability and clinical application in psychopathology. Clinical Neurophysiol. 30(4): 211-231.
22. Polich, J. (1997). On the relationship between EEG and P300: individual differences, aging, and ultradian rhythms. International Journal of Psychophysiology. 26(1–3): 299-317.
23. Donchin, E., Coles, M.G.H. (1988). Is the P300 component a manifestation of context updating?. Brain Behav. Sci. 11: 357-374.
24. Okawa, M., Matousek, M., Petersen, I. (1984) Spontaneous vigilance fluctuations in the daytime. Psychophysiology. 21: 207-211.
25. Tsuji, Y. Kobayashi, T. (1988). Short and long ultradian EEG components in daytime arousal. Electroenceph. Clin. Neurophysiol. 70: 110-117.
26. Lesiuk, T. (2005). The effect of music listening on work performance. Psychology of Music. 33(2): 173-191.
27. Warm JS, Parasuraman R, Matthews G. (2008). Vigilance requires hard mental work and is stressful. Hum. Factors. 50(3): 433-41.
28. Parasuraman, R., & Hancock, P. A. (2004). Neuroergonomics – Harnessing the power of brain science for human factors and ergonomics. Bulletin of the Human Factors and Ergonomics Society, December 2.
29. Parasuraman, R. (2011). Neuroergonomics: Brain, Cognition, and Performance at Work. Current Directions in Psychological Science, 20(3), 181–186. doi:10.1177/0963721411409176
30. Parasuraman, R. (2005). Neuroergonomics: An overview of research and applications. Foundations of Augmented Cognition Vol 11, 839–840.
31. Weber, R., Tamborini, R., Westcott‐Baker, A., & Kantor, B. (2009). Theorizing flow and media enjoyment as cognitive synchronization of attentional and reward networks. Communication Theory, 19(4), 397-422.
32. Ericsson, K. A., Krampe, R. Th., & Tesch-Römer, C. (1993). The role of deliberate practice in the acquisition of expert performance. Psychological Review, 100(3), 363-406.
33. Parasuraman, R., Nestor, P., & Greenwood, P. (1989). Sustained-attention capacity in young and older adults. Psychology and Aging, 4(3), 339-345.
34. Baumann, N., & Scheffer, D. (2011). Seeking flow in the achievement domain: The achievement flow motive behind flow experience. Motivation and Emotion,35(3), 267-284.
Bibliography
– Kandel, E. R., Schwartz, J. H., & Jessel, T. M. (1991). Principles of Neural Science. (3rd Edit.) Norwalk, CT. Appleton & Lange.
– Wade, C. & Tavris, C. (1993). Psychology. (3rd Edit.) New York, NY. HarperCollins College Publishers.
– Carlson, N. R. (1995). Foundations of Physiological Psychology. (3rd Edit.) Needham Heights, MA. Allyn & Bacon.
– Griffin, J.E. & Ojeda, S. R. (Eds.). (1996). Textbook of Endocrine Physiology. (3rd Edit.) New York, NY. Oxford University Press.
– Kandel, E. R., Schwartz, J. H., & Jessel, T. M. (1991). Principles of Neural Science. (3rd Edit.) Norwalk, CT. Appleton & Lange.
– Wade, C. & Tavris, C. (1993). Psychology. (3rd Edit.) New York, NY. HarperCollins College Publishers.
– Carlson, N. R. (1995). Foundations of Physiological Psychology. (3rd Edit.) Needham Heights, MA. Allyn & Bacon.
– Griffin, J.E. & Ojeda, S. R. (Eds.). (1996). Textbook of Endocrine Physiology. (3rd Edit.) New York, NY. Oxford University Press.
Comments
Post a Comment